Numaferm GmbH uses Teclen® Lyoprotect® Bags to avoid Fly-out of Peptides


About Numaferm GmbH
Founded in 2017, Numaferm GmbH has become a first-rate, trusted partner in the field of recombinant expression of peptides and proteins.
The team of about 30 experts, including 15 Ph. D. level scientists, has collectively cumulated more than 100 years of expert knowledge in the field of process development and production. The generous laboratory facility is equipped with state-of-the-art technology and hence offers excellent conditions for the development of fast, cost-efficient and resource-friendly production processes for the manufacture of high-purity products.
Within the framework of technology platforms developed by Numaferm, such as NumaswitchTM, cells of the Escherichia coli bacterium are reprogrammed to produce the desired peptide / protein. These patented processes rank among the most successful ones in the market, also because the costs are only one-tenth of the chemical synthesis. In addition, Numaferm has recently commercialized its first own enzyme product NumacutTM TEV protease. This means that the innovation and development work, which has so far only been utilized inside the company, is now also accessible to clients and partners.
NumacutTM TEV protease is a highly active, optimized variant of the Tabacco Etch Virus (TEV) protease with widened substrate tolerance and high stability. In order to ensure a high storage stability and a more environmentally friendly and cost-efficient transport, NumacutTM TEV protease is lyophilized and can subsequently be shipped at room temperature.
Initial Situation
In the present production process, enzyme products, such as NumacutTM TEV protease, were to be shipped as lyophilisate to the customers. Top priority was a consistent product quality. The liquid formulation was to be filled into vials suitable for shipment, with subsequent lyophilization.
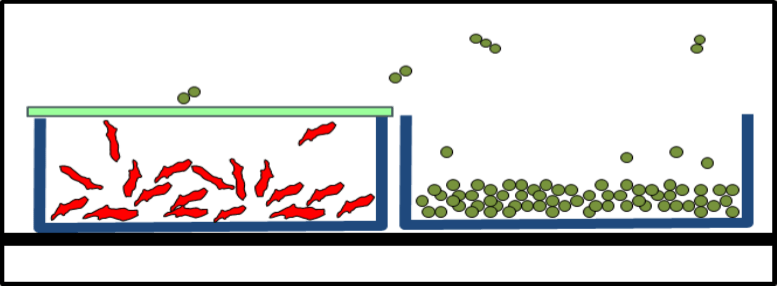
During lyophilization handling, fly-out and concomitant product loss as well as any contamination of the products or environment were to be excluded. After lyophilization, the vials were to be closed with their stoppers.
The Solution: Lyoprotect® Bag
Dr. Christian Schwarz got in touch with Teclen GmbH  in order to find out how Lyoprotect® Bags can be used for lyophilization in vials. The customizable bag sizes were a big asset, as well as the Lyoprotect® Membrane’s minimal impact on the drying conditions in comparison to an open vial, and of course also the excellent barrier properties of the Lyoprotect® Bag.
in order to find out how Lyoprotect® Bags can be used for lyophilization in vials. The customizable bag sizes were a big asset, as well as the Lyoprotect® Membrane’s minimal impact on the drying conditions in comparison to an open vial, and of course also the excellent barrier properties of the Lyoprotect® Bag.
The vials are filled in a storage box and are then placed into the Lyoprotect® Bag, which is already in place in the freeze-drier. The bag is then closed and secured with the closing stick. When the freeze-drying process is finished, the vials can be securely closed by means of an auxiliary plate.
Numaferm GmbH has successfully been using Lyoprotect® Bags in the lyophilization of liquid peptide formulations in preparative quantities for quite some time now.

CEO at Numaferm
“Using the customized Lyoprotect® Bags improves not only product handling but also staff safety with regard to fly-out. The various Lyoprotect® Bag sizes allow for maximum flexibility with different batch sizes and fillings – both equally important for our service and product business.“
NumacutTM is a trademark of Numaferm GmbH.
More information about the product of Numaferm GmbH: NumacutTM TEV Protease
Check out more details about the Lyoprotect® bags and the process with vials!
Contact us for questions or your unbinding quote!






 About Lactosan GmbH & Co KG
About Lactosan GmbH & Co KG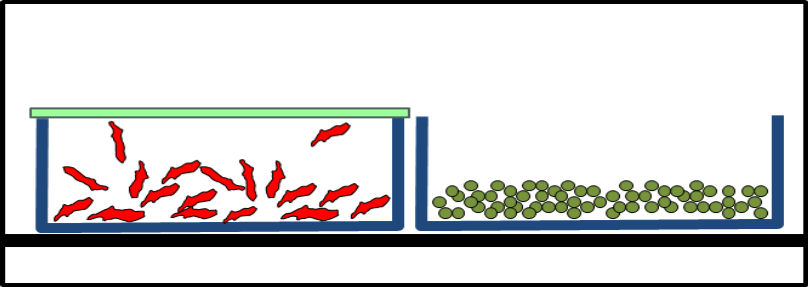




 “We’d already tried out several sterilization bags, but always had to compromise on the drying parameters. With Lyoprotect® our drying philosophy of the Single-Step-Cycle can now be realized much better – we also consider the Lyoprotect® system fit for GMP production of lyophilized drugs.” (Dr. Andreas Schütz, Managing Director at
“We’d already tried out several sterilization bags, but always had to compromise on the drying parameters. With Lyoprotect® our drying philosophy of the Single-Step-Cycle can now be realized much better – we also consider the Lyoprotect® system fit for GMP production of lyophilized drugs.” (Dr. Andreas Schütz, Managing Director at